Your pathway to streamlined medical device development. With pre-approved MDR 2a and FDA compliance, our platform slashes development costs. Seamlessly integrate innovative solutions and accelerate time-to-market. Join the future of digital health with myoncare.
No matter whether you need an advanced infrastructure for an existing solution or want to realize a new idea quickly and cost-effectively.1
.svg)
Class IIa medical device
ISO27001 & ISO 13485 certified QMS of ONCARE
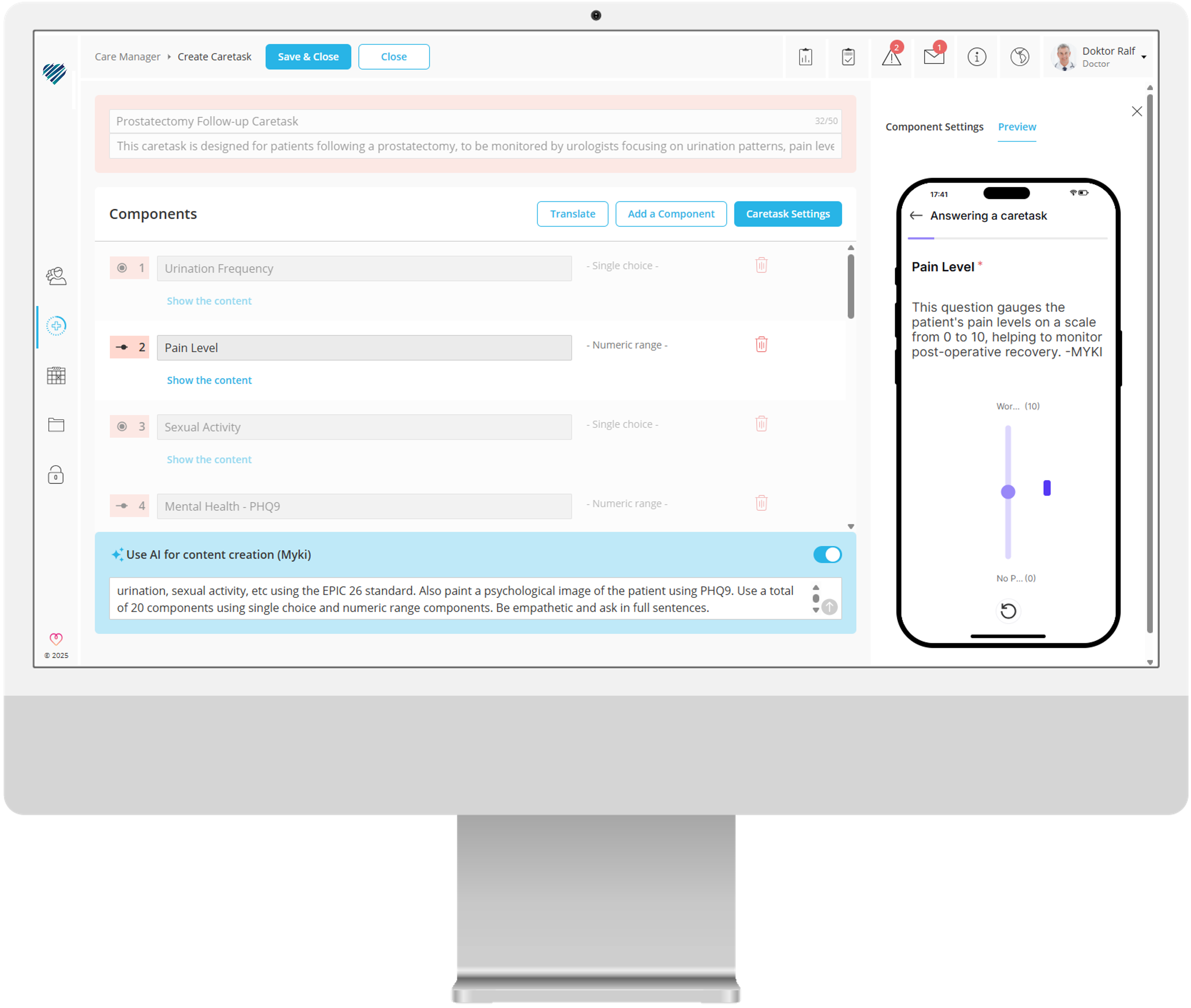
No-Code Content Management System
Numerous features and automation options
Continuous further development since 2015

Hosted on servers in Germany
Already numerous successful projects
Interfaces to Withings, Apple Health and Google Fit




Medical device software that previously belonged to Class I will, with a few exceptions, fall into at least Class IIa in future. This primarily affects software that is intended to provide information that is used to make decisions for diagnostic or therapeutic purposes.
Manufacturers must establish a quality management system certified in accordance with DIN EN ISO 13485.
Strict requirements apply to clinical trials, market surveillance and incident reporting.
Detailed technical documentation is essential, including comprehensive safety and performance certificates.
Whether you want to design your own digital program from scratch or offer your patients digital support with minimal effort - we have the right solution.

Maximum flexibility for your project. Create and combine your own content and manage patients via the innovative dashboard.

Digital health with minimal effort. Integrate your patients into an existing health program from our partner myon.clinic.

Our team will discuss your project idea with you. Book your non-binding consultation appointment now!
We use Calendly as our appointment system provider which needs you to allow all the cookies and refresh the page orclick here. (By clicking on the link you will be redirected to the external page Calendly.com).