
- EN
- DE
Associations
You develop standards – with myoncare, you translate them into scalable, digital care pathways, connect members, and make care outcomes measurable.

.svg)

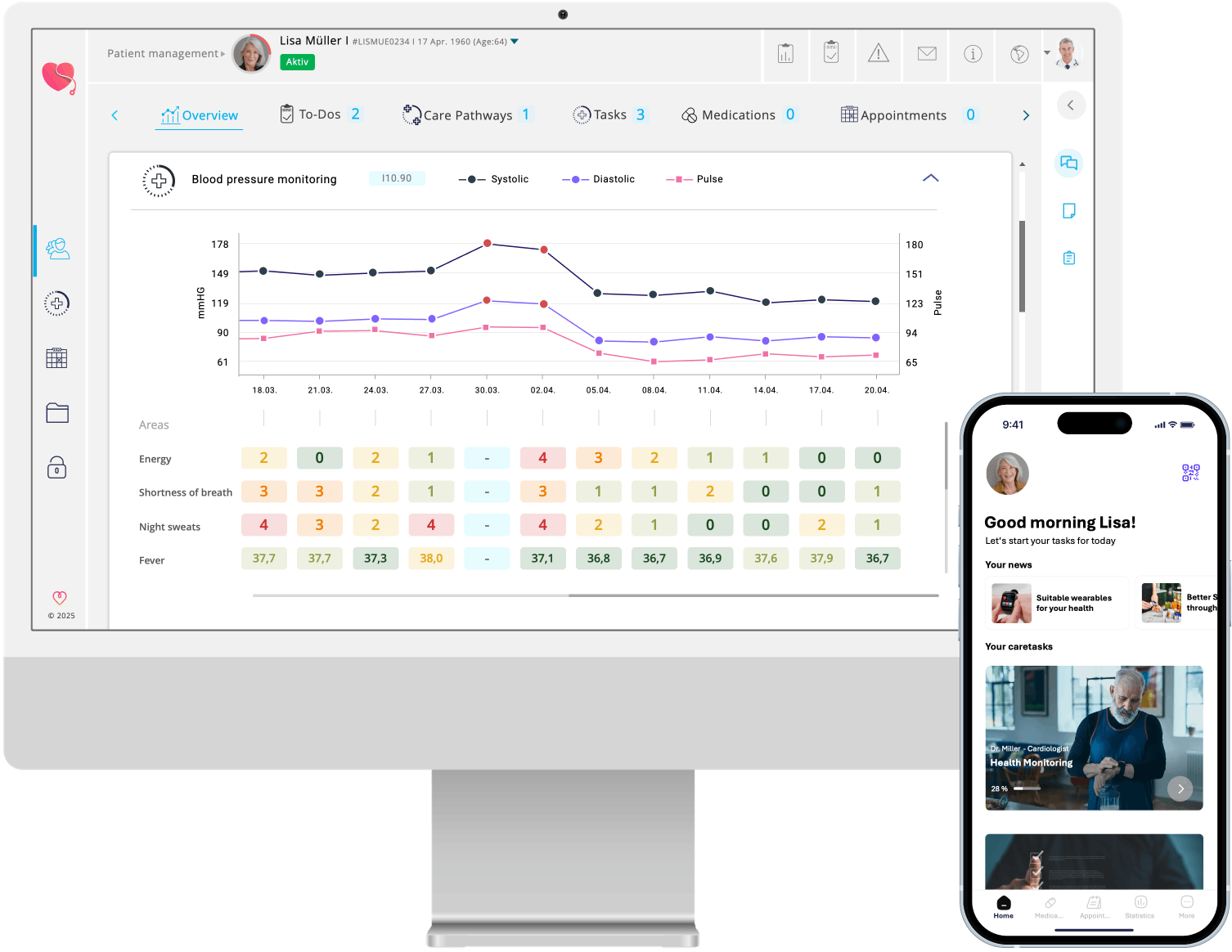
With myoncare, you can translate your guidelines into consistent, digital journeys—including structured data collection, member networking, and evaluations for quality and registration.
.png)
.png)
Create and maintain guideline-based pathways for your indications—from initial contact to follow-up and aftercare—that can be used directly by practices and clinics.

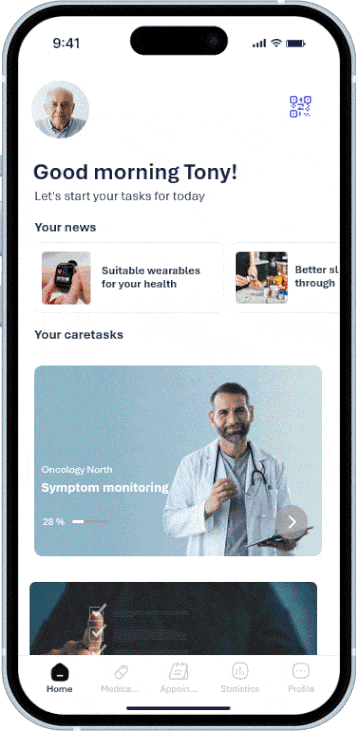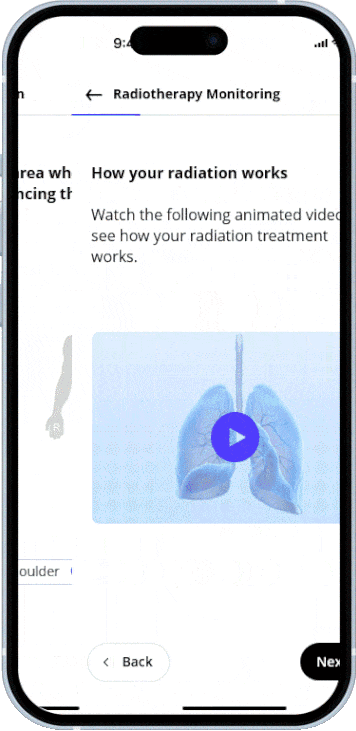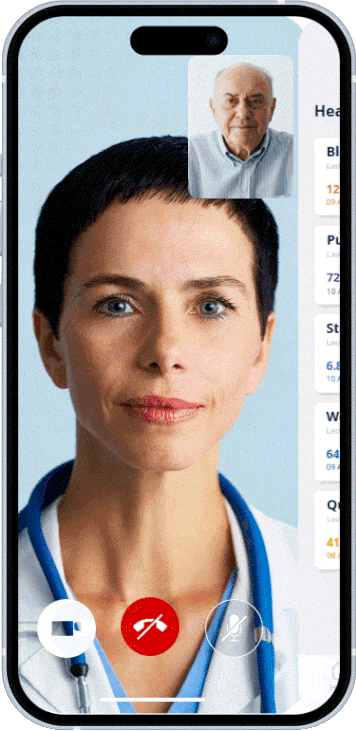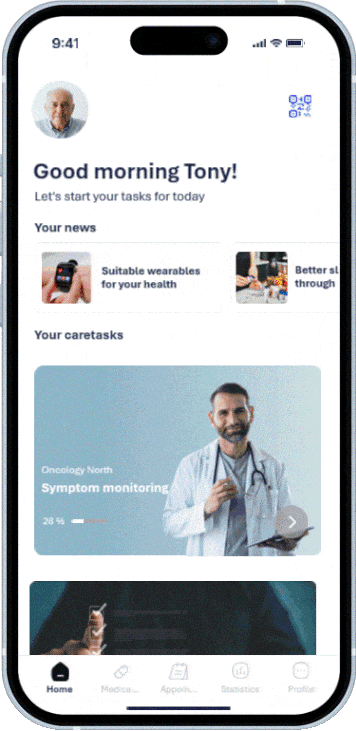
Member practices and clinics can work on a shared platform, share structured information, and implement standards uniformly.
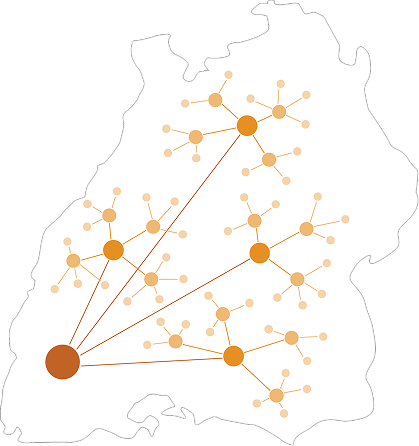
PROMs, scores, events, and histories can be collected in a standardized manner—as a basis for registries, quality reports, and health policy work.

Tailored use cases for your association – from standard programs to collaborations.
Provide indication-specific standard pathways (e.g., oncology, cardiology, diabetology) that your member centers can activate for their patients.
Your digital paths automatically feed into a quality assurance register—with uniform data points and exportable evaluations.
Your courses and guidelines can be linked to real care data from myoncare, making them useful for both teaching and practical purposes.
Use the digital programs as a basis for projects with clinics, industry, or cost bearers—including scalable implementation.
Trusted By






















The content management system is the heart of myoncare. Using the CMS, content can be easily created, stored with automation rules and combined into treatment paths. The user interface of the CMS has been designed to be particularly intuitive so that it can also be easily operated by users without programming knowledge.