
- EN
- DE
Security & Quality
Data protection and IT security are paramount. By design we safeguard sensitive information, ensuring compliance with the industry regulations. With a strong focus on confidentiality and integrity, users can trust myoncare to prioritize the security of their data at every step.

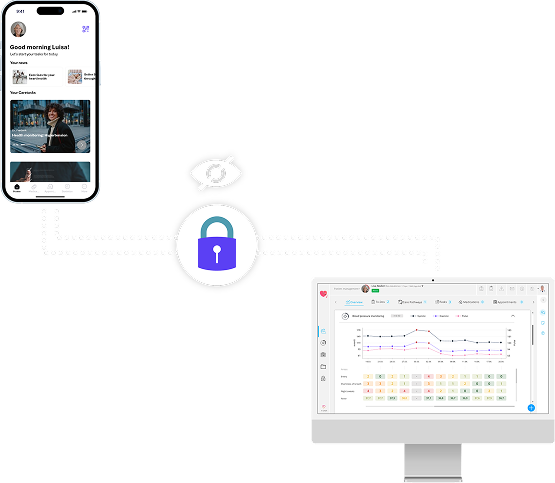

Participants can initiate a private transaction
Only anonymized data is included in the block
The block is sent to the network of nodes
The nodes validate the transaction
The block is added to the existing blockchain
Other participants have no insight into the details of the transaction.







Trusted By






















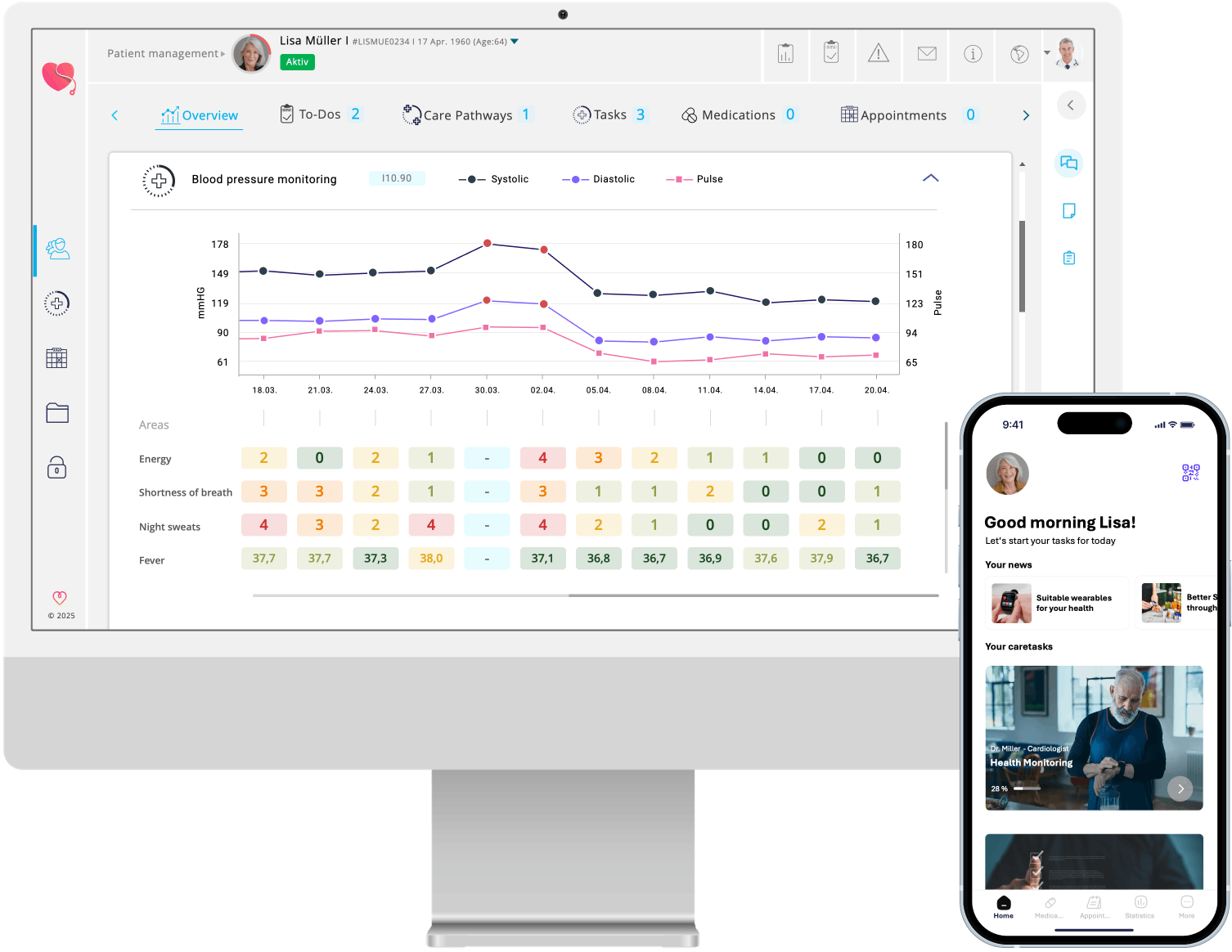
One platform connecting patients, care teams, and data. myoncare automates risk assessments, health monitoring, reminders, education, and telecommunication - all in one compliant infrastructure.
to get the tech, compliance, and infrastructure right
– so you don’t have to.

with our ai-powered content management system & pathway builder.
Choose from numerous modules to create customized solutions:

Designed to meet the highest standards for compliance, interoperability, flexibility, and research-driven collaboration.
The content management system is the heart of myoncare. Using the CMS, content can be easily created, stored with automation rules and combined into treatment paths. The user interface of the CMS has been designed to be particularly intuitive so that it can also be easily operated by users without programming knowledge.