
- EN
- DE
Manufacturers
Our digital health platform empowers you to create personalized digital companion solutions that can support medication efficacy and improve data accessibility.

.svg)
.svg)
Create digital therapy and product companions to support doctors and patients from screening and diagnosis through to long-term therapy support.
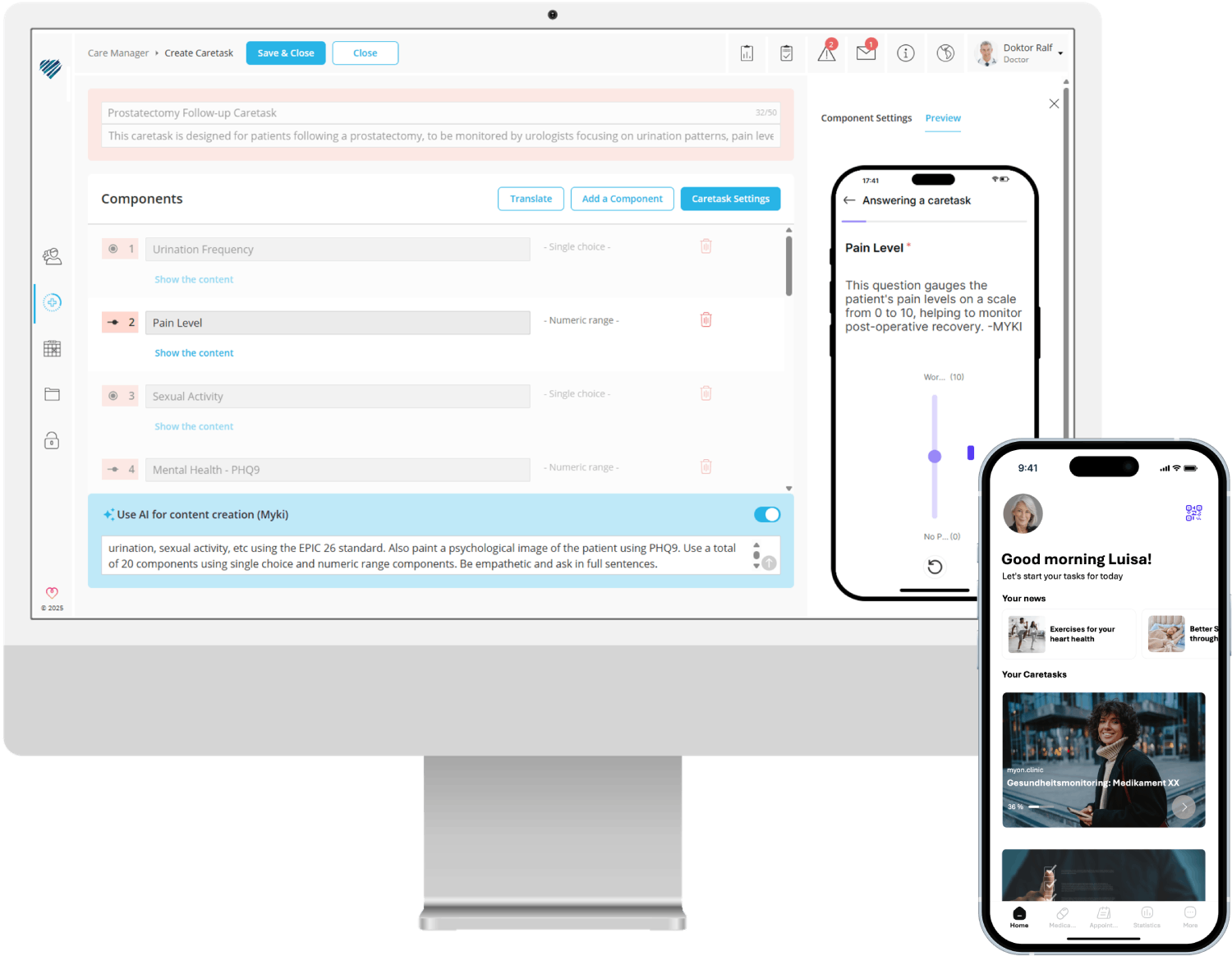
seamless patient support with real-time monitoring of symptoms and promotion of adherence through reminders and educational content.
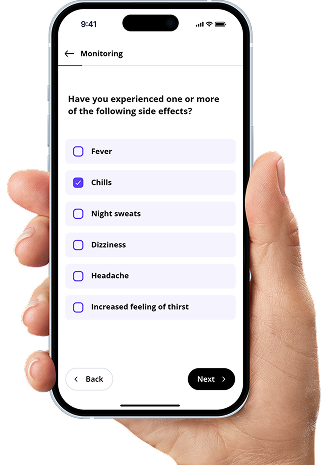
Connect relevant service providers involved in the care of patients treated with your products, enabling structured support throughout the entire patient journey.
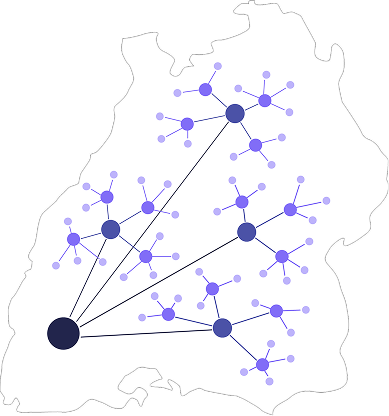
myoncare powers large‑scale, anonymized real‑world data collection to drive studies and meet post‑market surveillance needs.

Empower physicians and patients to provide structured support throughout the entire patient journey, obtain anonymized real-world data for studies and post-market surveillance, and automate your study processes—all in one data-driven ecosystem.
Streamline patient care within interdisciplinary networks and manage diseases smarter through data-driven interventions
Guide patients step-by-step through complex treatments with symptom tracking, personalized content and reminders.
Transform routine care data into actionable insights to drive research, reimbursement, and quality-based outcomes.
Digitally manage study participants, ePROs, and visit schedules to ensure compliance and data integrity across sites.
Trusted By






















The content management system is the heart of myoncare. Using the CMS, content can be easily created, stored with automation rules and combined into treatment paths. The user interface of the CMS has been designed to be particularly intuitive so that it can also be easily operated by users without programming knowledge.